| Product name |
Infant Sleep Positioners (Warning) |
| Description |
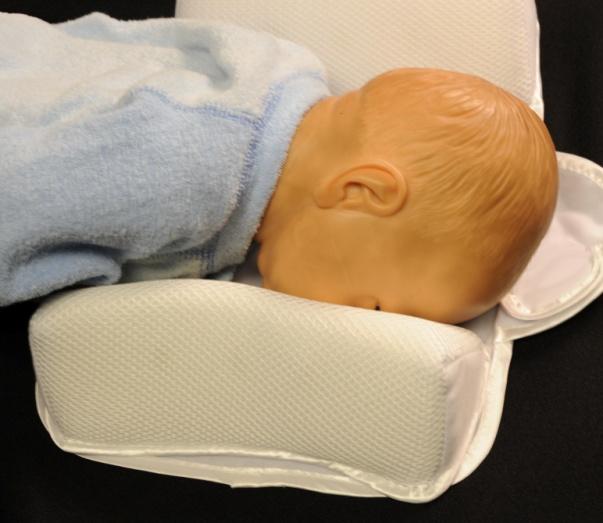
The U.S. Consumer Product Safety Commission (CPSC) and the U.S. Food and Drug Administration (FDA) warns consumers to stop using infant sleep Positioners. The two main types of infant sleep positioners are flat mats with side bolsters or inclined (wedge) mats with side bolsters. Both types of sleep positioners typically claim to help keep infants on their backs and reduce the risk of Sudden Infant Death Syndrome (SIDS). |
| Problem |
Infants can suffocate in sleep positioners or become trapped and suffocate between a sleep positioner and the side of a crib or bassinet. -- The FDA has never cleared an infant sleep positioner to prevent or reduce the risk of SIDS. In addition, CPSC and the FDA are unaware of any scientific studies demonstrating that infant positioners prevent SIDS or are proven to prevent suffocation or other life-threatening harm. The American Academy of Pediatrics does not support the use of any sleep positioner to prevent SIDS. -- Sleep positioners also typically claim to do one or all of the following: aid in food digestion to ease colic or the symptoms of gastroesophageal reflux disease (GERD); and prevent flat head syndrome (plagiocephaly). In light of the new safety data, FDA believes any benefit from using these devices to ease GERD or prevent plagiocephaly is outweighted by the risk of suffocation. |
| Distribution |
|
| Company |
|
| Dates sold |
|
| Date posted |
10/08/2010 |
| Image |
|
|
|