| Product name |
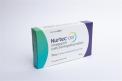
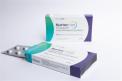
NurtecŪ ODT (rimegepant) orally disintegrating tablets, 75mg 8-Unit Dose blister pack |
| Description |
This recall involves prescription drugs Nurtec ODT 75 mg orally disintegrating tablets sold in cartons containing one blister card of 8 tablets. The tablets are in a non-child resistant blister card packaged in a carton that includes the name of the product, dosage strength, NDC number and expiration date. The dosage strength and expiration date are printed or stamped on the blister card. The recall includes the following: NurtecŪ ODT (rimegepant) 75mg 8-Unit Dose blister pack 72618-3000-2. All dates through 6/2026 |
| Problem |
The recalled prescription drugs must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children. |
| Distribution |
Pharmacies nationwide as a prescribed medicine. The prices of the product varied based on health insurance terms and other factors. |
| Company |
Pfizer 800-879-3477; webpages www.pfizer.com/contact or online at www.Nurtec.com/PackagingUpdate or www.Nurtec.com |
| Dates sold |
December 2021 through March 2023 |
| Date posted |
03/16/2023 |
| Additional information |
For additional information, visit the U.S. Consumer Product Safety Commission's news release at https://www.cpsc.gov/Recalls/2023/Pfizer-Recalls-Nurtec-ODT-Prescription-Drugs-Due-to-Failure-to-Meet-Child-Resistant-Packaging-Requirement-Risk-of-Poisoning |
| Image |
|
|
|