Product Details
| Product name | Kroger Aspirin, 300 count bottles and Ibuprofen, 160 count bottles | |
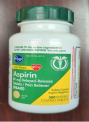
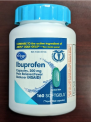
| Description | This recall involves the Kroger brand aspirin and ibuprofen over-the-counter drugs. For the aspirin product, the green and yellow label states Kroger, Low Dose, Aspirin, 81 mg Delayed-Release Tablets / Pain Reliever, 300 enteric coated tablets. The bottle has a green continuous thread gear closure. For the ibuprofen product, the blue and white label states Kroger, Ibuprofen, Capsules, 200 mg Pain Reliever / Fever Reducer, 160 softgels. The bottle has a blue continuous thread gear closure. The following UPC and Lot numbers listed in the table are included in this recall and can be found on the label on the back of the bottle. Item/UPC (Lot numbers) - Aspirin/0004126001295 (A077J, F032H, F035H, J011H, and K031H), and Ibuprofen/0004126001298 (FH1163, C11044, C11047, C11064, C11065, C11079, and C11084). | |
| Problem | The recalled over-the-counter products contain the regulated substances aspirin and ibuprofen which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children. | |
| Distribution | (See news release for full list) | |
| Company | Kroger 800-576-4377 (800-KRO-GERS); web page www.kroger.com/i/recall-alerts, or www.kroger.com or www.timecaplabs.com | |
| Dates sold | August 2021 through March 2022; July 2021 through March 2022 | |
| Date posted | 06/17/2022 | |
| Additional information | For additional information, visit the U.S. Consumer Product Safety Commission's news release at https://www.cpsc.gov/Recalls/2022/Time-Cap-Labs-Recalls-Kroger-Brand-Aspirin-and-Ibuprofen-Due-to-Failure-to-Meet-Child-Resistant-Packaging-Requirement-Risk-of-Poisoning | |
| Image |
|
|