| Product name |
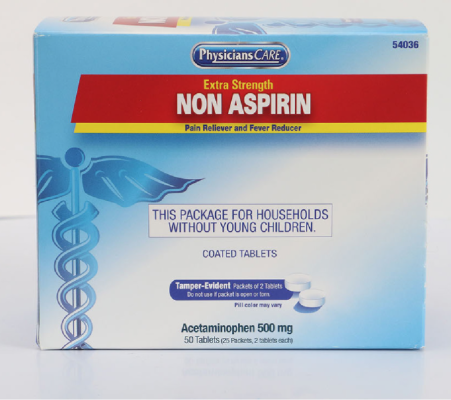
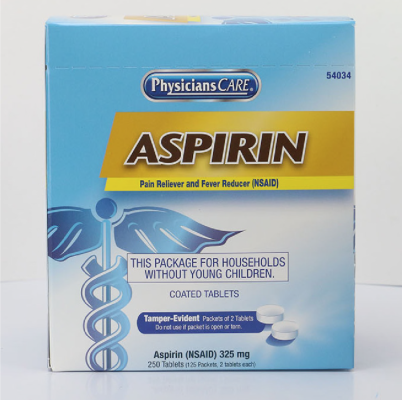
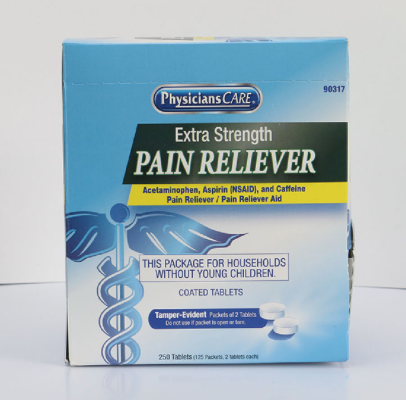
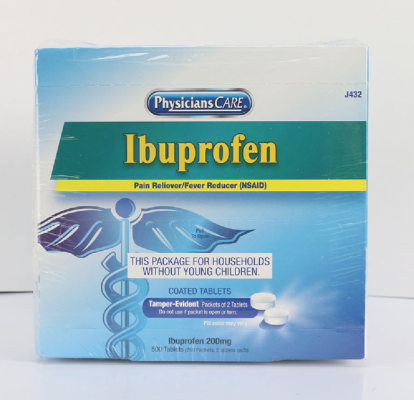
PhysiciansCare brand Aspirin, Extra Strength Non Aspirin, Extra Strength Pain Reliever, Ibuprofen, Medication Station, and Multi-Pack |
| Description |
This recall involves the PhysiciansCare brand Extra Strength Non Aspirin, Aspirin, Extra Strength Pain Reliever, Ibuprofen, Medication Station, and Multi-Pack over-the-counter drugs. The products contain aspirin, acetaminophen, or ibuprofen. They are packaged in cardboard boxes of 50, 100, 250, and 500 tablets per box. A list of recalled products can be found on the U.S. Consumer Product Safety Commission's news release at https://www.cpsc.gov/Recalls/2022/Acme-United-Corporation-Recalls-PhysiciansCare-Brand-Over-the-Counter-Drugs-Due-to-Failure-to-Meet-Child-Resistant-Packaging-Requirement-Risk-of-Poisoning-Recall-Alert |
| Problem |
The recalled over-the-counter products contain regulated substances (aspirin, acetaminophen, or ibuprofen) which must be in child resistant packaging when being used in the household as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children. |
| Distribution |
Amazon.com and FSAstore.com for between $5 and $50. |
| Company |
Acme United 888-520-2199; web page www.recallrtr.com/acmeunitedotc or at www.acmeunited.com |
| Dates sold |
February 2014 through June 2021 |
| Date posted |
03/18/2022 |
| Additional information |
For more information, visit the U.S. Consumer Product Safety Commission's news release at https://www.cpsc.gov/Recalls/2022/Acme-United-Corporation-Recalls-PhysiciansCare-Brand-Over-the-Counter-Drugs-Due-to-Failure-to-Meet-Child-Resistant-Packaging-Requirement-Risk-of-Poisoning-Recall-Alert |
| Image |
|
|
|