| Product name |
Over-the-counter drugs |
| Description |
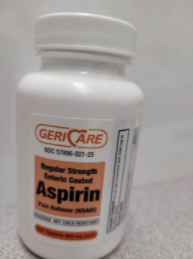
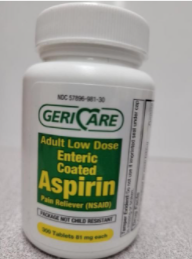
This recall involves bottles of Geri-Care Brand over-the-counter acetaminophen and aspirin. The acetaminophen is 500mg with 1,000 tablets in the bottle. The aspirin was sold in 81mg with 300 and 1,000 tablets in the bottle and 325mg with 250 and 1,000 tablets in the bottle. Product (Count). Extra Strength Acetaminophen 500mg Tablets (1,000), Regular Strength Enteric Coated Aspirin 325mg Tablets (250), Regular Strength Enteric Coated Aspirin 325mg Tablets (1,000), Adult Low Dose Enteric Coated Aspirin 81mg Tablets (300), and Adult Low Dose Enteric Coated Aspirin 81mg Tablets (1,000). |
| Problem |
The over-the-counter drug products contain regulated substances which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children. |
| Distribution |
Online at amazon.com, simplymedical.com, drugsupplystore.com, heypharma.com, otcsuperstore.com, blowoutmedical.com, vitamincoveusa.com, simplymedical.com, silverrodrx.com, zoro.com, healthproductsforyou.com, earthturns.com, cleanitsupply.com, herbspro.com, stomabags.com, ebay.com, atcmedical.com, and bettymillls.com between $2 and $10. |
| Company |
Geri-Care Pharmaceuticals 800-540-3765; www.gericarepharm.com/recalls/ or www.gericarepharm.com |
| Dates sold |
August 2021 |
| Date posted |
01/26/2022 |
| Additional information |
For additional information, visit the U.S. Consumer Product Safety Commission's news release at https://www.cpsc.gov/Recalls/2022/Geri-Care-Pharmaceuticals-Recalls-Over-the-Counter-Drugs-Due-to-Failure-to-Meet-Child-Resistant-Packaging-Requirement-Risk-of-Poisoning |
| Image |
|
|
|